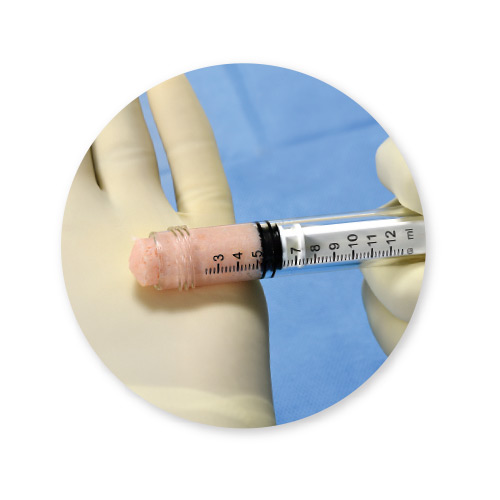
VIA Form+™ is the next generation allograft containing key elements ideal for bone formation and offers a variety of potential clinical applications, including the spine, upper extremity, foot and ankle, oral and maxillofacial, and orthopedic oncology. The allograft is packaged in an easy-to-use syringe with minimal preparation time of under 15 minutes.
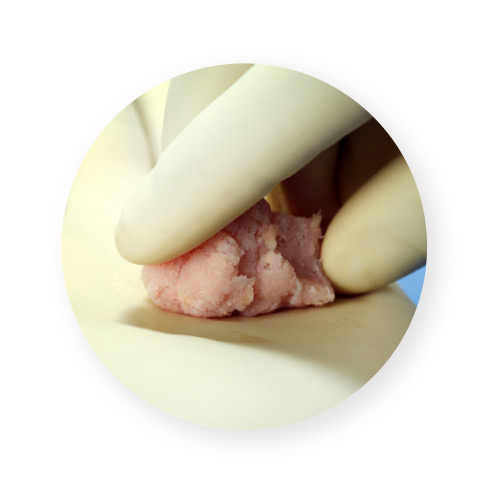
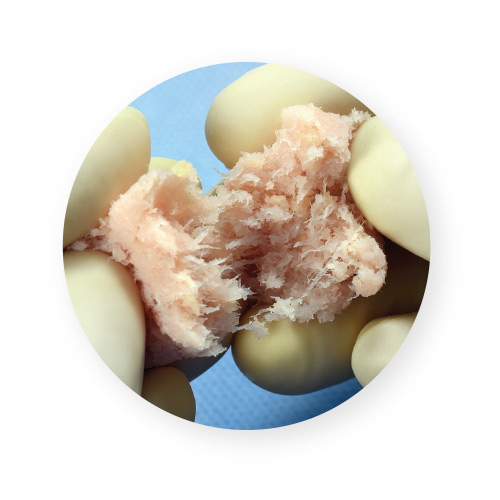
VIA Form+ provides an osteoconductive bone scaffold composed of mineralized cancellous bone along with demineralized cortical fibers. Bone fibers offer superior osteoconductivity when compared to powder due to the increased ability for cells to migrate along fibers, creating “cellular highways” for bone formation.2 In contrast, particulate-based demineralized bone matrices (DBMs) have gaps between the particles that osteoblasts cannot always bridge across.2 The demineralized cortical fibers are supplemented with cancellous chips to deliver a 100% human-derived product that mimics the particulate structure of native bone.

2 Martin GJ Jr, Boden SD, Titus L, Scarborough NL, “New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis.”, Spine. 1999 Apr 1;24(7):637-45.

